Abstract
Introduction
Newly diagnosed multiple myeloma (NDMM) patients who achieve and maintain minimal residual disease (MRD) negativity demonstrate clinical benefit with prolonged progression-free survival and overall survival. Based on available data showing MRD negativity with standard dose KRD (36 mg/m2) approximating 40% (Korde JamaOnc 2015), we designed a MRD response-adapted treatment study for NDMM, where the number of treatment cycles is determined based on MRD status, instead of the traditional paradigm of fixed number of cycles followed by autologous hematopoietic cell transplantation (AHCT). We integrated a flow-based MRD driven platform in this phase I/II study evaluating higher doses of twice-weekly carfilzomib (Car) (45 and 56 mg/m2) in combination with lenalidomide (Len) and dexamethasone (Dex).
Methods
Eligible NDMM patients were given escalating doses of Car (45 and 56 mg/m2), Len, and Dex in a single arm, phase I standard 3+3 schema design, based on dose-limiting toxicities (DLTs) occurring in cycle 1. Treatment consisted of 28-day cycles with Car 20/45 mg/m2 or 20/56 mg/m2 - days 1, 2, 8, 9 15 and 16; Len 25 mg - days 1-21; and Dex 40 mg weekly cycles 1-4, 20 mg after cycle 4. AHCT eligible patients underwent stem cell collection after 6 cycles, and then continue with protocol therapy. Patients achieving MRD negative status (serum, urine, and bone marrow with 10-color flow) received 2 additional cycles from the time of conversion and then stop therapy. Patients with less than an MRD negative response after any cycle continued therapy until treatment completion (max 12 cycles), disease progression, or unacceptable toxicity. The primary endpoint of the phase II study was to determine the MRD negative rate at the MTD dose, using a Simon's optimal two-stage design. Herein, we have updated results on phase I and phase II portions of the study with a median follow-up of 20.7 months (1.4-31.1). For available data, we present MFC and NGS MRD platforms.
Results
Twenty-nine patients have enrolled onto study between October 2016 - June 2018, with 18 in phase 1 and stage I of phase II and 11 in stage II of phase II, thus completing target accrual. There were 16 males, 13 females, median age 61 (43-75) years. Baseline characteristics included 18(62%) ISS-I, 9(31%) ISS-II, and 2(7%) ISS-III, and 7(24%) patients high risk FISH (t(4,14), t(14,16), p53 deletion). There were no DLTs within the first cycle that met protocol criteria (0/3 patients in 20/45 mg/m2 cohort and 0/6 patients in 20/56 mg/m2). The MTD chosen was 20/56 mg/m2, and an additional 20 patients were enrolled. Three patients came off study (56 mg/m2 cohort): one due to MI (during C2); one due to intolerable rash (during C2); and one due to personal preference (during C2). Among all 29 patients, grade 3/4 non-hematologic toxicities included 6(21%) rash, 5(17%) electrolyte disturbances, 4(14%) infections, 3(10%) GI, 2(7%) cardiopulmonary, 2(7%) VTE, 2(7%) mood, 2(7%) cataract, and 1(3%) hyperglycemia, and grade 3/4 hematologic toxicities included 12(41%) lymphopenia, 2(7%) leukopenia, 1(3%) neutropenia, and 1(3%) thrombocytopenia. Ten patients had 13 SAEs.
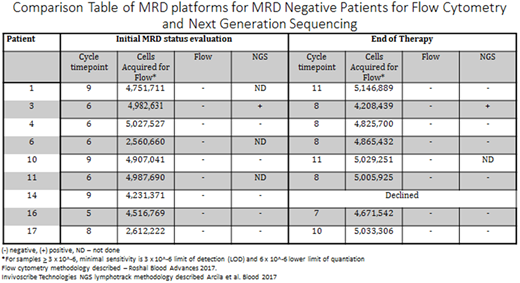
For the 15 patients completing protocol therapy, a median number of 11 (7-12) cycles were delivered, and best responses include 9(60%) sCR/CR MRD neg and 6(40%) obtaining VGPR. Among patients reaching sCR/CR MRD neg status, the median time to reach was 8 (5-9) cycles. Among the eleven patients currently remaining on study, 7 have received at least 1 cycle of therapy (response eligible) and best responses thus far, included 1(14%) sCR/CR MRD pending, 4(57%) VGPR, and 2(29%) PR with a median number of 4 (1-8) cycles delivered. Table comparison of MRD platforms shown in sCR/CR patients. Among patients that remain on study, median 20.7 months, no patients have progressed and all remain alive. One patient that came off study due to personal preference during cycle 2 achieved a PR, and has progressed since.
Conclusion
In this phase I/II clinical trial assessing higher doses of twice-weekly Car dosing in combination with Len and Dex, we established MTD 56 mg/m2 and demonstrated a MRD platform using multi-parametric flow cytometry can be successfully used to tailor individualized treatment plans. Higher doses of twice weekly Car (45 and 56 mg/m2) in combination with Len and Dex, resulted in rapid and deep responses with approximately 60% MRD negative rate, and a safety profile similar to KRD standard dose (Car 36 mg/m2).
Korde:Amgen: Research Funding. Mailankody:Janssen: Research Funding; Physician Education Resource: Honoraria; Takeda: Research Funding; Juno: Research Funding. Hassoun:Oncopeptides AB: Research Funding. Lesokhin:Janssen: Research Funding; Squibb: Consultancy, Honoraria; Genentech: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Serametrix, inc.: Patents & Royalties: Royalties; Takeda: Consultancy, Honoraria. Smith:Celgene: Consultancy, Patents & Royalties: CAR T cell therapies for MM, Research Funding. Arcila:Invivoscribe, Inc.: Consultancy, Honoraria. Ho:Invivoscribe, Inc.: Honoraria. Landgren:Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Research Funding; Merck: Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; Karyopharm: Consultancy; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal